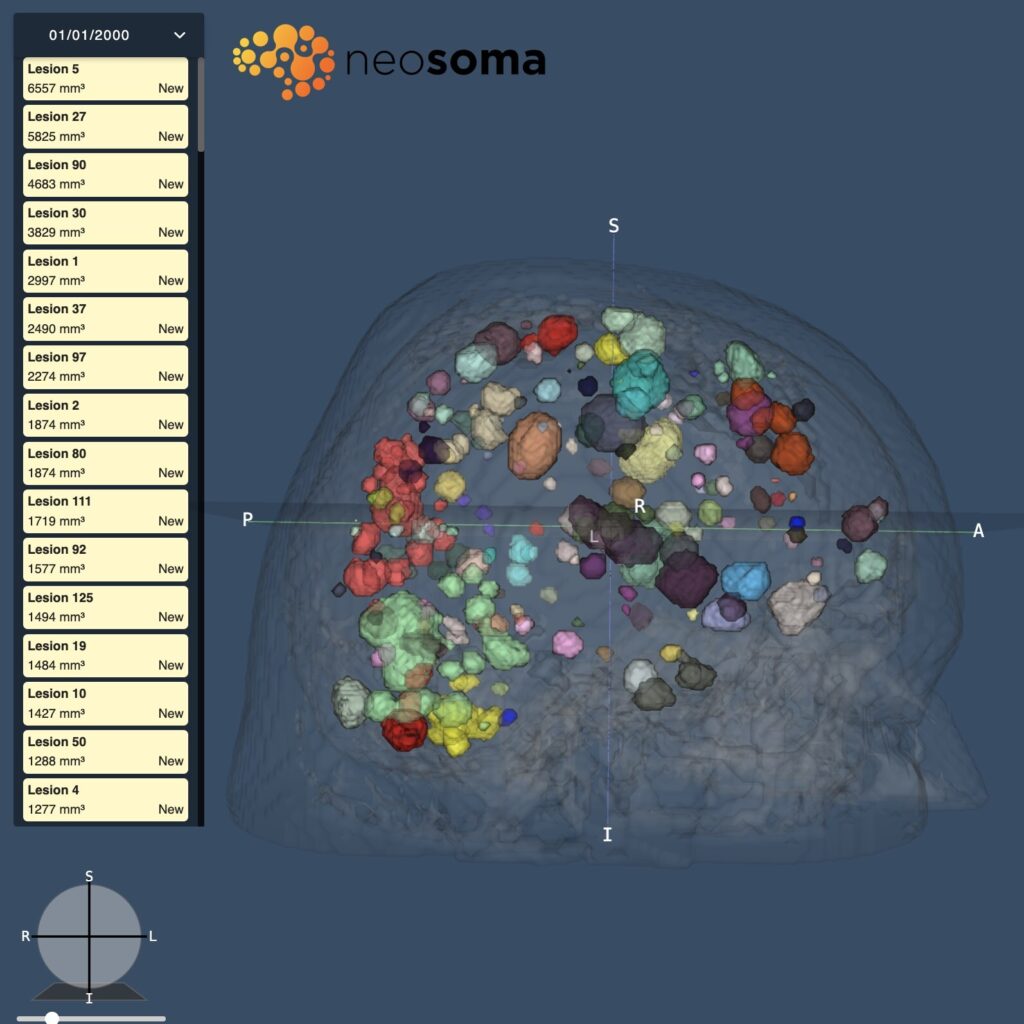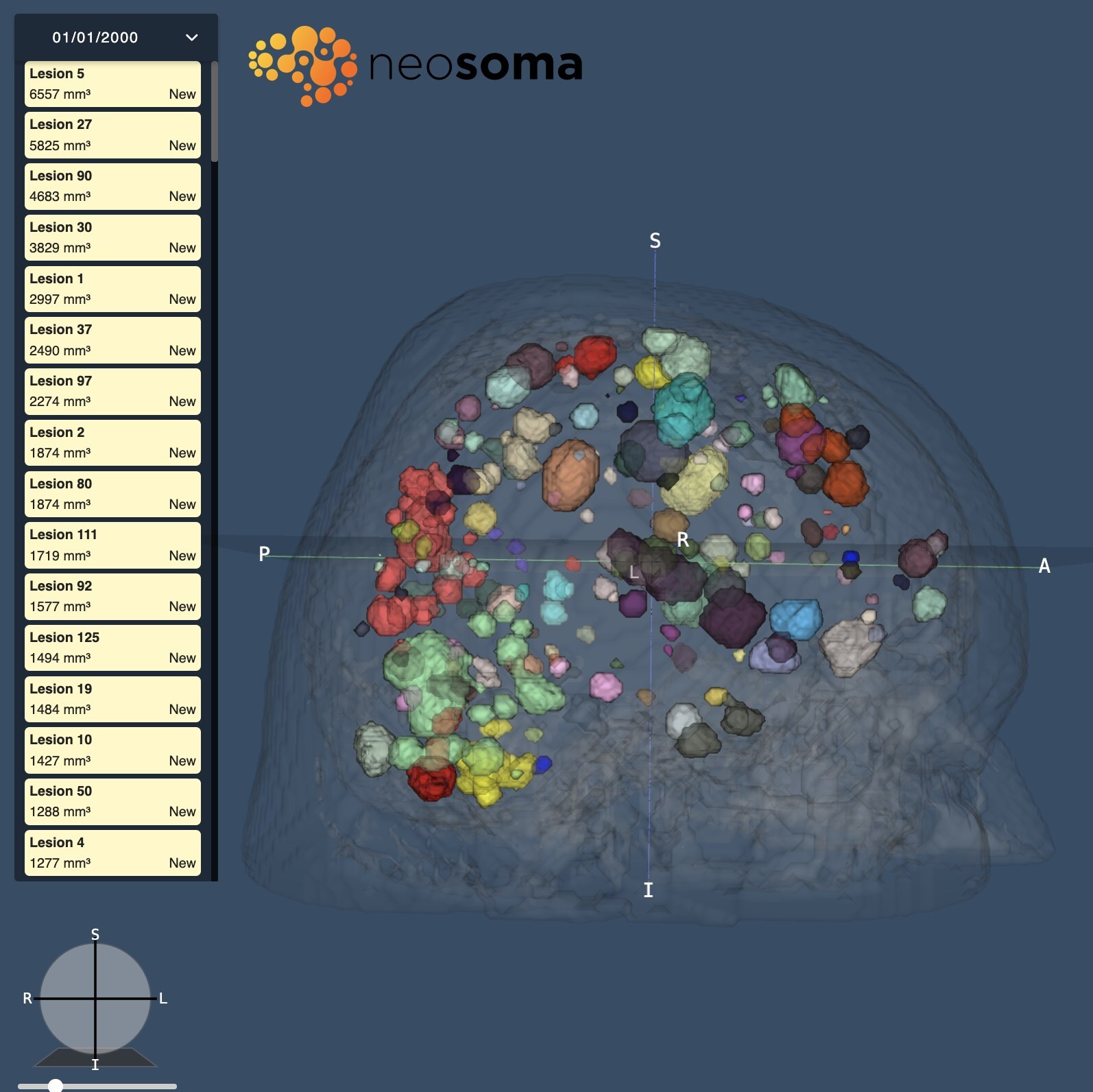
Neosoma, a best-in-class provider of AI-based brain tumor analysis medical software, receives FDA 510(k) clearance for its Brain Mets product. This clearance ushers in a new era in the assessment of brain metastases, providing novel tumor insights to physicians and researchers in…